Integrative Translations Blog
Kerilyn Sappington, Chinese to English Medical Translator
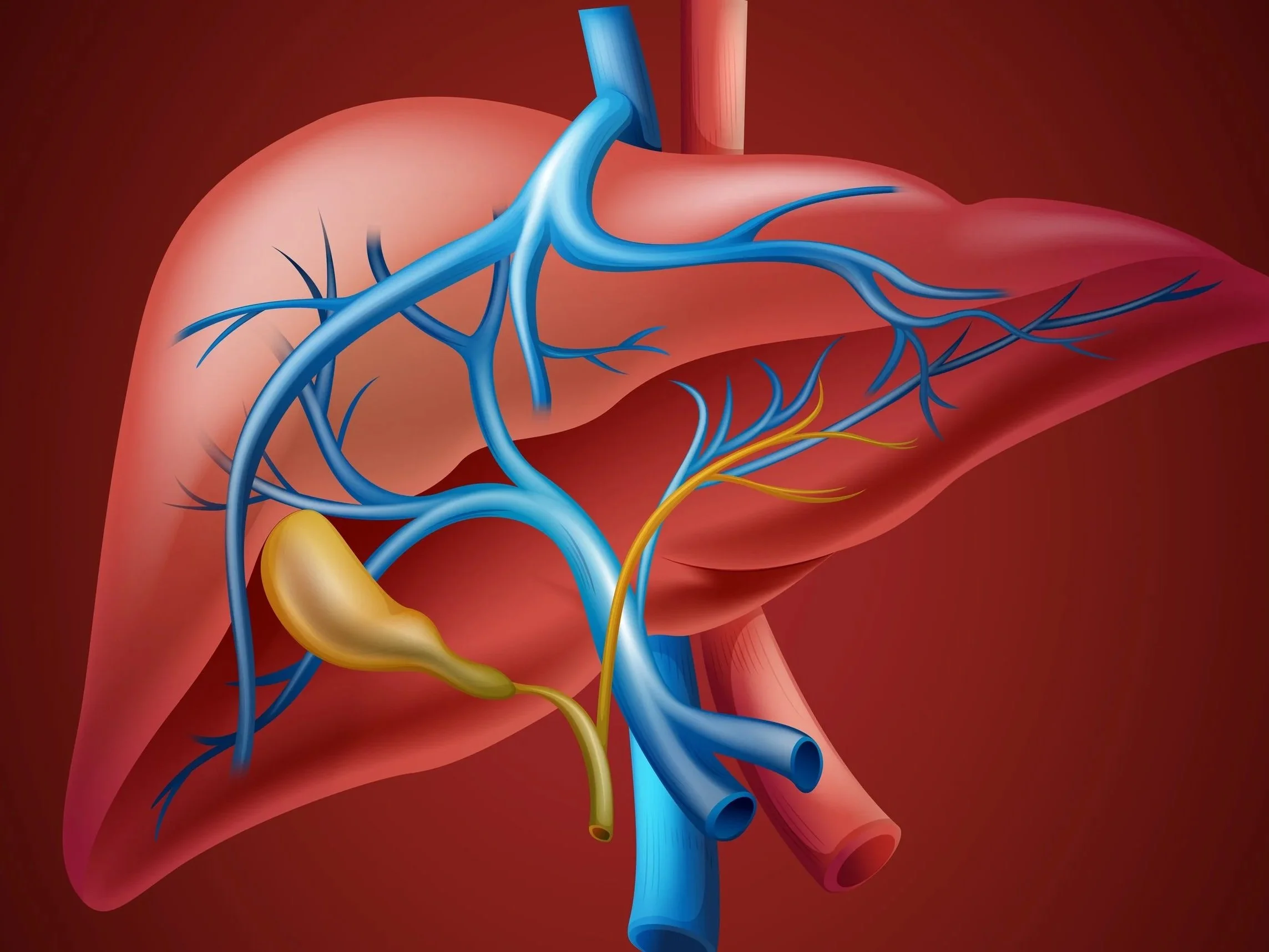
Herbs to Treat Drug-Induced Liver Injury
Chinese herbs alleviate liver disease, reduce severity of liver damage, and suppress the pathways and mechanisms of liver injury, among other hepatorestorative effects. Active ingredients include polyphenol compounds, flavonoid compounds, saponins, organic acids, terpenoid compounds, phenylpropanoids, polysaccharides, and alkaloids.
Basil, lemongrass, ginseng, and licorice root are among the Chinese herbs with hepatoprotective and hepatorestorative effects.
Snake Venoms in Drug Discovery
Animal venoms for defense and predation are not a single toxin but are instead a complex mix of components (proteins, peptides, and enzymes) with specific biological and pharmacological activities. New drug development is one of the greatest challenges in the pharmaceutical industry today, and animal venoms have emerged as a source of therapeutic drugs. Over the past 75 years, potential therapeutic agents have been extracted and isolated from the toxins of plants, animals, and microorganisms, and in the past few decades several drugs have been isolated or derived from snake venom proteins.
Is This Document Korean, Chinese, or Japanese?
Chinese, Japanese, and Korean all use characters, sometimes they use the same characters. While modern Japanese is a mix of kanji (Chinese characters) and the katakana and hiragana syllabaries, most of the time Korean is written in the Hangul alphabet, although I have seen older Korean documents written with Chinese characters.
Integrative Medicine and the Treatment of Knee Osteoarthritis
What is the role of alternative medicine in the treatment of knee osteoarthritis? Let's take a brief look at two recent articles. The Annals of Internal Medicine published Effectiveness of Curcuma longa Extract for the Treatment of Symptoms and Effusion–Synovitis of Knee Osteoarthritis (Wang, Jones, et al.) in 2020.
Yew Tree Bark: Or How Paclitaxel Became the Most Well-Known Naturally Sourced Cancer Drug
Paclitaxel (trade name Taxol) is derived from the bark of the Pacific yew tree (Taxus brevifolia) and is used to treat breast, lung, and ovarian cancer.
Behavioral Cardiology
In medical treatment today, heart health requires more than blood pressure control or the right pharmacologic therapy. Doctors advise lifestyle modifications in the form of diet and exercise yet may encounter resistance.