Snake Venoms in Drug Discovery
“The global animal venom library is a collection of more than 40 million peptides of which only a small fraction have been identified.”
A COMPLEX COCKTAIL
Animal venoms for defense and predation are not a single toxin but are instead a complex mix of components (proteins, peptides, and enzymes) with specific biological and pharmacological activities. New drug development is one of the greatest challenges in the pharmaceutical industry today, and animal venoms have emerged as a source of therapeutic drugs. Over the past 75 years, potential therapeutic agents have been extracted and isolated from the toxins of plants, animals, and microorganisms, and in the past few decades several drugs have been isolated or derived from snake venom proteins.
TRADITIONAL MEDICINE
For thousands of years, snake venom has been employed in Ayurveda, homeopathy, and traditional medicine. The snake was the god of medicine in ancient Greece. In Ayurveda, cobra venom was used to treat joint pain, inflammation, and arthritis, and traditional Chinese medicine has extensively incorporated snake blood and bile. The 17th-century Italian naturalist Felice Fontana was among the first in the modern era to discover the coagulant effects of snake venom.
LEAD MOLECULES
As biotechnology has advanced, the number of drugs designed from snake venom has increased. Produced by specialized exocrine glands connected to the fangs by ducts, venoms vary across species and even within the same snake specimen. Venoms are composed of about 100 to 500 pharmacologically active compounds with an estimated 10 to 50 million natural compounds available for drug discovery. Researchers view these active compounds as natural biology libraries with immense potential for the identification of new drugs and, unlike synthetic compound libraries, the compounds in natural libraries are known to be biologically active. These molecules have been fine-tuned during evolution and natural selection for target selectivity, low immunogenicity, and high stability.
Neurotoxins leading to acute neuromuscular paralysis, morbidity, and mortality were first purified from snake venoms approximately 50 years ago. Most neurotoxins act on the peripheral nervous system; the skeletal neuromuscular junction is a frequent target. Cytotoxic snake venoms target specific cell sites and interact with lipoproteins present in plasma membrane to shrink cells. The cytotoxic effects of snake venom components may degrade and destroy tumor cells.
Venoms interact with mammalian proteins to disrupt the central and peripheral nervous systems, the blood coagulation cascade, the cardiovascular and neuromuscular systems, and the general homeostasis state. Phospholipase A2 (PLA2), serine proteases, metalloproteinases, lectins, l-amino-acid oxidases, bradykinin-potentiating factors, natriuretic factors, and integrin antagonists in venom possess natural pharmacological actions and may induce neurotoxicity, myotoxicity, cytotoxicity, hematotoxicity, and antimicrobial activity with applications in cardiovascular medicine, antibacterial therapies, pain management, neurological disorders, and anticancer treatment.
There are snake venom proteins that mimic the effects of endogenous kallikrein, natriuretic peptides, and vascular endothelial growth factors. Hyaluronidases, endoglycosidases present in almost all snake venoms, are considered "spreading factors" that damage the extracellular matrix at the site of the snake bite and lead to severe morbidity. Hyaluronidases both damage local tissue and facilitate the distribution of other toxins. PLA2 enzymes alter membrane fluidity causing it to become permeable.
CARDIOVASCULAR MEDICINE
Snake venoms provide an abundant source of lead molecules that affect the cardiovascular system, which makes them pharmaceutically significant. Catalytically active and inactive PLA2s reduce blood pressure through the production of arachidonic acid, a precursor of cyclooxygenase metabolites. The low molecular weight fibrinolytic enzymes jararafibrases I, II, III, and IV isolated from the venom of Bothrops jararaca exhibit hemorrhagic activity. Proteolytic enzymes catalyze the digestion of tissue proteins and peptides into amino acids. Metalloproteases and serine proteases affect the hemostatic system, and peptide toxins isolated from venoms target ion channels, membrane receptors, and components of the hemostatic system with high selectivity and affinity.
Snake venom metalloproteinases (SVMPs) are known mainly for their pro-hemorrhagic activities. They have the potential to interfere with the hemostatic system, however SVMPs have also been associated with fibrinogenolysis and fibrinolysis, prothrombin activation, platelet aggregation inhibition, and inactivation of blood serine proteinase inhibitors. Because SVMPs degrade the main components of the capillary basement membrane, they cause the escape of blood content from the intravascular space to surrounding tissues and enable the distribution of venom. Thrombin-like enzymes isolated from venom possess coagulant activities similar to thrombin but they are not inhibited by heparin and do not activate blood coagulation factor XIII. The hematotoxic components of snake venom can be categorized into coagulant, anticoagulant, and fibrinolytic factors, and more than 100 components act on the hemostatic system through different mechanisms. The snake venoms of the Viperidae and Crotalidae families are a rich source of proteins and peptides that interact with the hemostatic system, resulting in characteristic post-envenomation hemorrhage and distribution of venom. Snake proteins that act on elements of the coagulant cascade and activate the coagulation system include activators of blood coagulation factors V, IX, and X and prothrombin activators. Activators of blood coagulation factor X have been isolated from the venom of several snake species. In the clotting process, factors V, IX, and X, along with calcium ions and phospholipids, activate prothrombin and induce fibrin clot formation. Snake venom toxins function as direct or indirect anticoagulants by inhibiting the clotting process. Because of their considerable impact on blood coagulation, snake venom activators may be a source of new therapeutic agents.
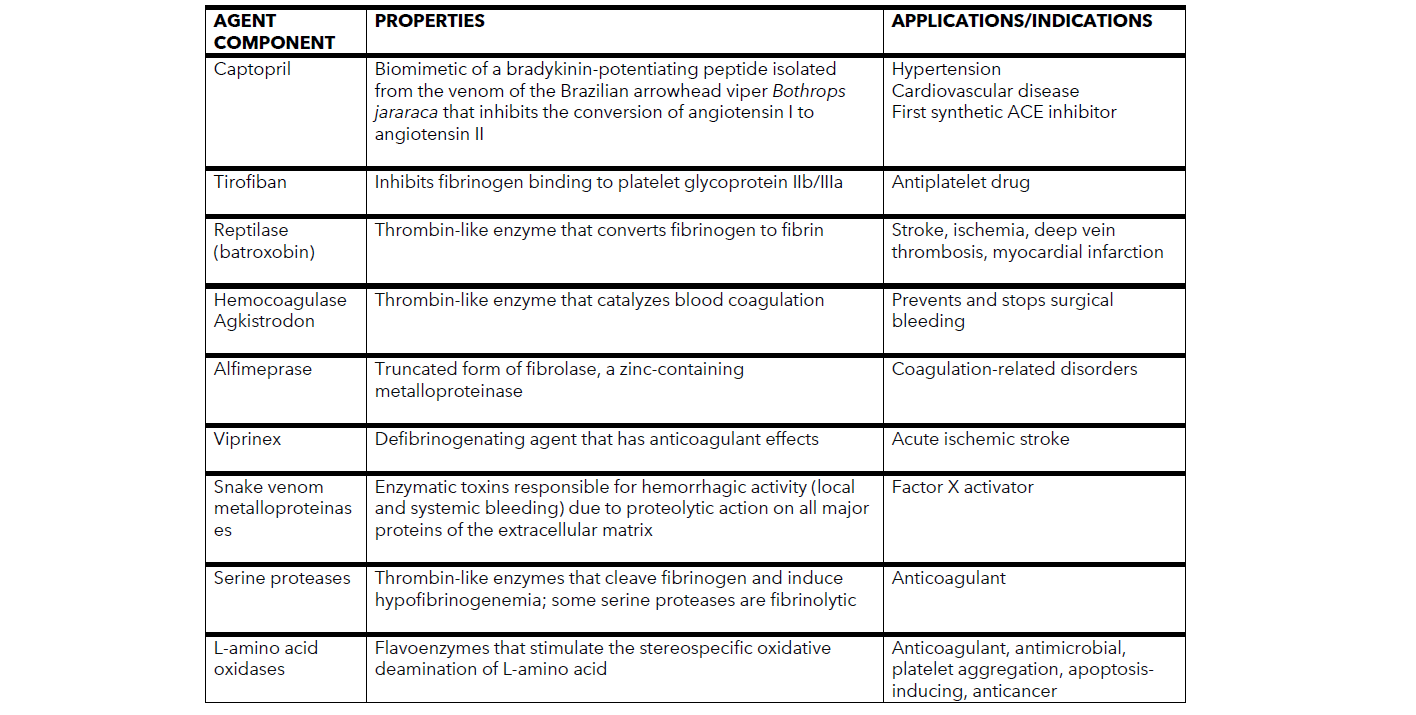
COMPONENTS OF SNAKE VENOM
CAPTOPRIL
The discovery of angiotensin-converting enzyme (ACE) inhibitors was a tremendous achievement in cardiovascular pharmacology. Captopril, the first ACE inhibitor approved for human use, was also the first drug successfully developed from a component of snake venom. A biomimetic of a bradykinin-potentiating peptide, captopril was isolated from the venom of the Brazilian arrowhead viper Bothrops jararaca. Captopril inhibits angiotensin-converting enzyme, which is responsible for the conversion of angiotensin I to angiotensin II. The drug was approved by the FDA in 1981 and its indications include hypertension, diabetic nephropathy, and heart failure.
Since the approval of captopril, snake venoms have become an important natural pharmacopeia of bioactive molecules that provide a good source of compounds for the development of new drugs. The development of captopril establishes the capacity of snake venom components to function as lead molecules in modern drug development. Only a small number of components have been identified, and continued advances in the drug discovery field are expected to yield new therapeutic leads from snake venoms.